PRIMAQUINE REVIEW
Primaquine revisited six decades after its discovery
Primaquine was first synthesized in 1946 in the USA.
Six decades have passed and primaquine is still the only transmission-blocking anti-malarial clinically available, displaying a marked activity against gametocytes [baby forms].
Primaquine is also effective against all exoerythrocytic forms [ones developing in the liver not blood] of the parasite and is used in conjunction with other antimalarials.
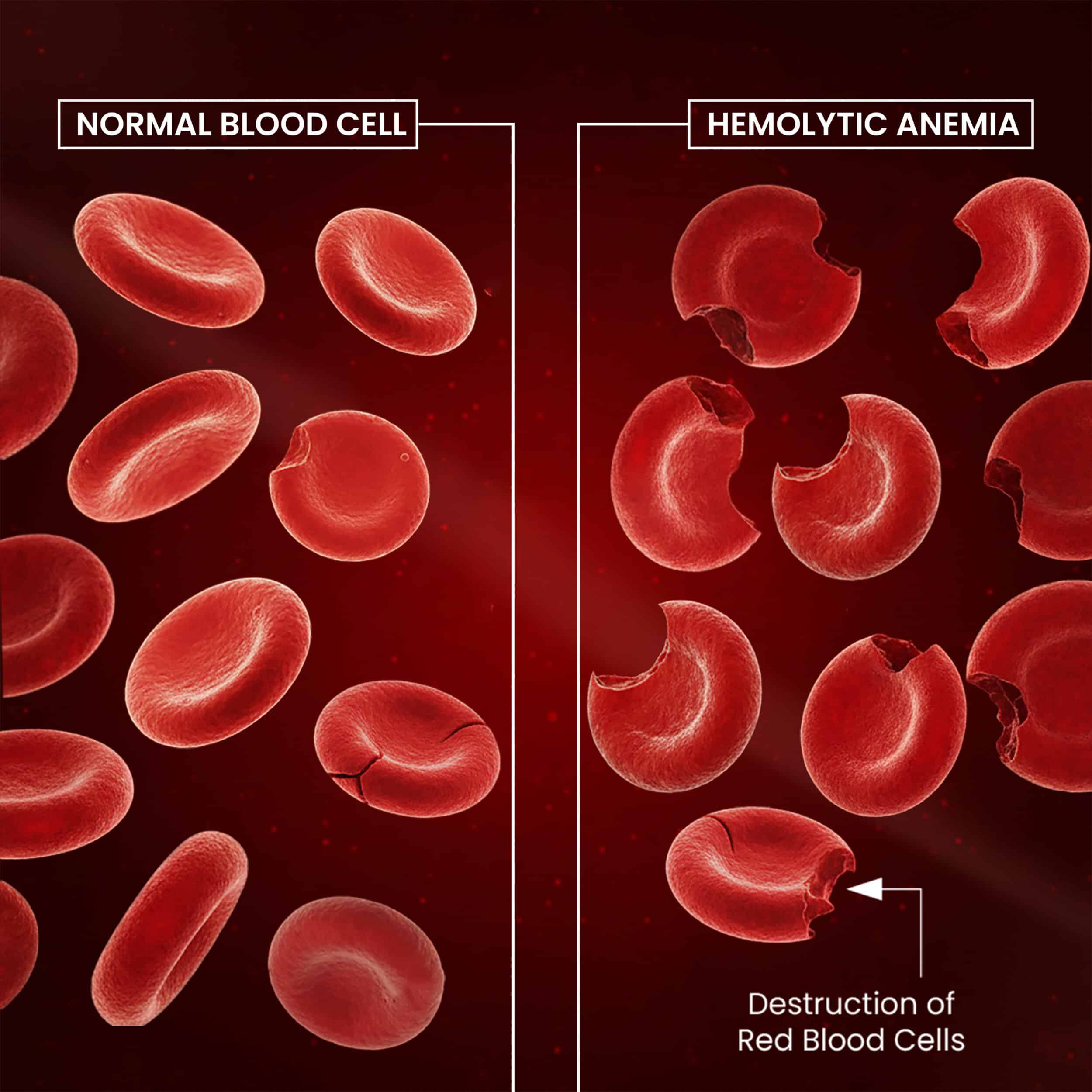
However, primaquine is often associated with serious adverse effects, in consequence of its toxic metabolites, that include hemolytic anemia.
Copyright 2026 James Schaller on all Unchanged-Free Use
Primaquine toxicity is aggravated in people deficient of 6-glucose phosphate dehydrogenase [ So checking a G6PD lab level to confirm you are normal is always required] or glutathione synthetase. [This latter substance is protective against reactive oxidative species and plays a critical role in detoxification processes.
Adverse effects are further amplified by the fact that primaquine must be repeatedly administered at high doses, due to its limited oral bioavailability. [We are looking at ways to improve this in other posts]
Over the last two decades, Medicinal Chemists have battled against primaquine’s disadvantages, while keeping or even improving its unequalled performance as an anti-malarial.
Presently, aablaquine and tafenoquine [available in the USA and in my definitive Healthcare Workers Babesia book in 2006. Over 16 years before it was FDA approved and prescribed and available] the two most promising primaquine analogues…. [Aablaquine is in our Feb. 10, 2026 blog]
Historical synopsis
Tropical diseases, normally confined to underdeveloped regions of the globe, have been traditionally neglected by the pharmaceutical industries and, consequently, seldom considered as hot matter capable of drawing the attention of top scientists, from chemists to physicians. This attitude was changed by force of historical events in some periods, such as the first half of the 20th century, when world-wide belligerency required western soldiers, fighting in tropical regions, to be protected.
Parasitic resistance
The establishment that parasitic resistance is occurring requires the demonstration that parasites are able to survive in vivo in the presence of an adequate therapeutic concentration of the drug system [117].
Several anti-malarial drugs are referenced as affected by the problem of resistance by Plasmodia, among which chloroquine is known to present severe resistance problems from both the deadliest P. falciparum and the second most concerning P. vivax strains [118], [119], [120].
Relevant primaquine metabolites
PQ is rapidly absorbed in the gastrointestinal tract and concentrated in the liver, brain, heart, lungs and skeletal muscle. The mean volume of distribution is 3 L/kg. It peaks in plasma within 1–3 h, at ∼70 mg/mL, and is rapidly excreted in urine, with a plasma half-life of 4–9 h [9].
PQ is primarily metabolised to carboxyprimaquine that is not accumulated in the body. PQ is also metabolised to a number of other identified and unidentified metabolites that are detectable in urine and plasma….
Final remarks
Primaquine may not be the anti-malarial drug with the best therapeutic profiles [121], [234], and several aspects of its biological action are yet to be discovered. However, primaquine is still the only transmission-blocking anti-malarial clinically useful and it goes on being used as the platform for developing novel anti-malarials with improved efficacy and reduced toxicity [21].
Good safety
tolerance and efficacy
key advantages in dosing requirements…
“make PQ an excellent drug.”
Source: Nuno Vale, Rui Moreira, Paula Gomes. European Journal of Medicinal Chemistry
Volume 44, Issue 3, March 2009, Pages 937-953. https://doi.org/10.1016/j.ejmech.2008.08.011
